Brief description of research and scholarly activity
 |
 |
The Carbon Based Nano-Materials (CBNM) research group forms part of the SARChI in Carbon Technology and Materials research group which is sponsored by National Research Foundation (NRF) of South Africa focusing on carbon-related materials in bulk and nano-structured levels for renewable energy applications.
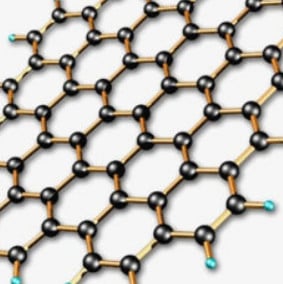
This dynamic group focuses on the investigation and optimization routes for synthesizing large area high quality chemical vapor deposition (CVD) graphene films, 3D graphene foam (GF) and highly porous activated carbon nanostructures along with their nano-composites for energy storage and sensing applications. In addition, some theoretical DFT calculations are done on co-doped graphene systems which are adopted for comparison with experimental findings.
Generally, the group is involved in optimizing the growth of graphene thin film to form single layer, bilayer and few layers through atmospheric pressure CVD growth. Secondly, transition metal oxides (tMOs) and hydroxides (tMOHs) synthesized using different methods (solvothermal, chemical bath deposition, microwave, green chemistry etc.) are incorporated into porous 3D graphene foam and activated carbon structures to obtain nano-composites suitable as active electrode materials for electrochemical capacitors and sensing applications.
 |
 |
|
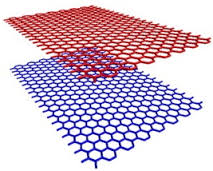
Bernal-stacked bilayer graphene, where half of the carbon atoms in the second layer sit on top of the empty centres of hexagons in the first layer, is an interesting material because of its tune-able bandgap of up to ca. 250 mV by an external electric field.
This unique property is attractive for fundamental research as well as applications where the ability to adjust the band gap is desired, such as tunnel field-effect transistors and tune-able laser diodes.
CVD technique is the synthesis method of choice for this special bilayer on Cu foil and Cu(Ni) alloys. The key point for bilayer graphene growth by CVD is to overcome the self-limiting nature of single layer graphene on Cu.
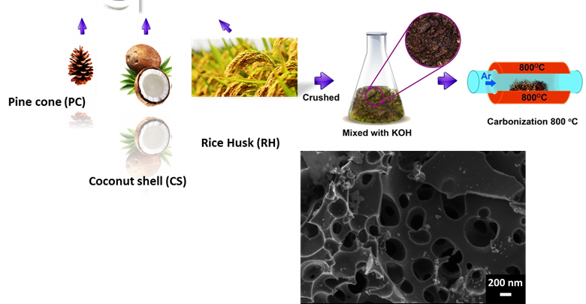
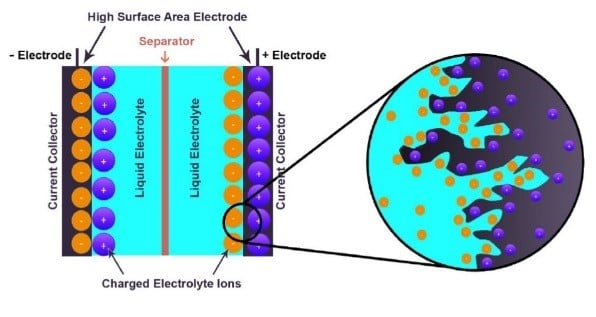
Electrochemical devices (batteries and capacitors) are the most promising energy storage systems. Electric double layer capacitors (EDLCs), also known as supercapacitors or ultracapacitors, have received a great attention because they can achieve a higher energy density than conventional capacitors and a better power density than batteries. A wide variety of different electrode materials and electrolytes are currently being investigated to improve on efficiency and practicality of EDLCs. Using graphene produced by chemical vapor deposition (CVD), as reference material, added with raw biomass carbon sources, we have been able to produce graphene-based activated carbon nanoporous material by carbonization and activation procedures. Recent work from the group have shown that the materials produced are good candidates for EDLC, with high micropore volume and good conductivity. Pseudocapacitive and Faradaic materials, which involve charge transfer between the electrode and electrolyte. Since the most important focus in the supercapacitors research is the improvement of energy density to be closer or comparable to that of batteries, the group also explores different electrolytes in order to improve on the potential window of supercapacitor devices and hence improvement of the energy density.







 Virtual Campus
Virtual Campus
Download the UP Mobile App